Scott Hensley
Stories
-

FDA authorizes 1st antiviral pill for Covid
In a highly anticipated decision, the Food and Drug Administration authorized Pfizer's Paxlovid as the first antiviral pill to treat Covid-19 at home.
-
CDC advisers vote to narrow use of J&J vaccine due to concerns about rare blood clots
The recommendation was prompted by the occurrence of a rare and sometimes fatal blood clotting problem known as TTS. More than 16 million people in the U.S. have received a shot of the J&J vaccine.
-

Pfizer data shows that its COVID-19 pill is effective against severe disease
The research finds that the medicine, called Paxlovid, was effective in preventing hospitalization and death when taken by people with mild to moderate illness within a few days of symptoms.
-

An FDA panel supports Merck COVID drug in mixed vote
If the Food and Drug Administration authorizes use of the drug, called molnupiravir, it would be the first oral COVID-19 treatment that could be taken at home.
-
CDC advisers back expansion of COVID boosters for all adults
Hours after the Food and Drug Administration authorized booster doses of COVID-19 vaccine for all adults 18 years and older, a panel of experts endorsed their use with a few caveats.
-
Moderna says new data supports its COVID vaccine for kids 6 to 11
Moderna says a study in kids 6 to 11 found two doses of the company's COVID-19 vaccine given 28 days apart produced a strong antibody response.
-
Pfizer-BioNTech COVID vaccine appears more than 90% effective in kids 5 to 11
The companies studied a 10 microgram vaccine dose in children 5 to 11, a third of the dose used for adults, to minimize side effects and because it still prompts a strong immune response.
-
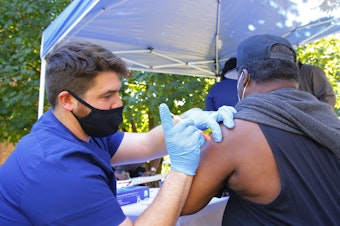
CDC advisers back rollout of COVID vaccine boosters from Moderna and J&J
A panel advising the Centers for Disease Control and Prevention also endorsed a mix-and-match approach to boosters that would be flexible for patients and health care providers.
-
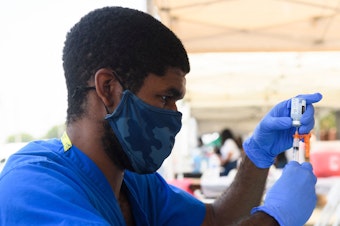
The FDA authorizes Moderna and J&J COVID vaccine boosters
The Food and Drug Administration also gave an OK to boosters that differ from the vaccine originally used to immunize people against COVID-19. A mix-and-match approach could ease the booster rollout.
-
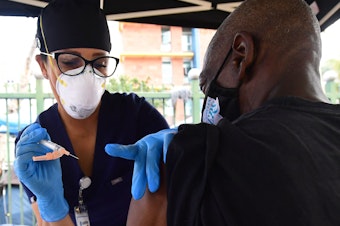
An FDA panel of experts backs J&J COVID vaccine booster
A panel of experts voted to recommend that the Food and Drug Administration authorize a booster dose of the Johnson & Johnson COVID vaccine at least two months after the first shot.